A well conducted audit will investigate every facet of your PSM system; both its structure and its implementation.
The audit itself should consisted of the following activities:
- Extensive review of PSM/RMP documentation provided by the facility
- Interviews with operating staff – The interviews with hourly personnel were conducted primarily to confirm written policies and procedures with actual practice.
- Tours of the covered processes – Physical inspections of operating unit equipment and observations of certain activities that happened to occur during the onsite period of the audit were conducted.
Audits have two major objectives.
- The first is to assess whether the management system in place adequately addresses all elements of the PSM Rule. This part of the audit attempts to discover fundamental design deficiencies that could compromise the effectiveness of a PSM program.
- The second objective is to assess whether the management system has been adequately implemented for every facility or process. Deficiencies discovered during this part of the audit may indicate a need for better communications or training, or may signal fundamental management problems.
What is your company’s compliance strategies and does your guideline address this? Does your management system understand your goals and deficiencies? Do your employees have the right cultural?
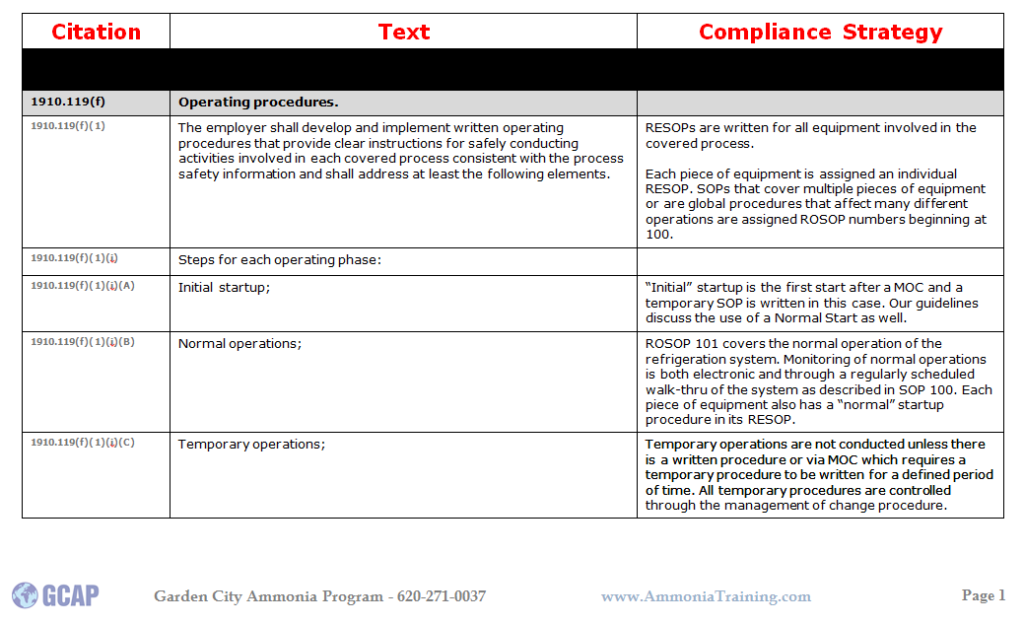
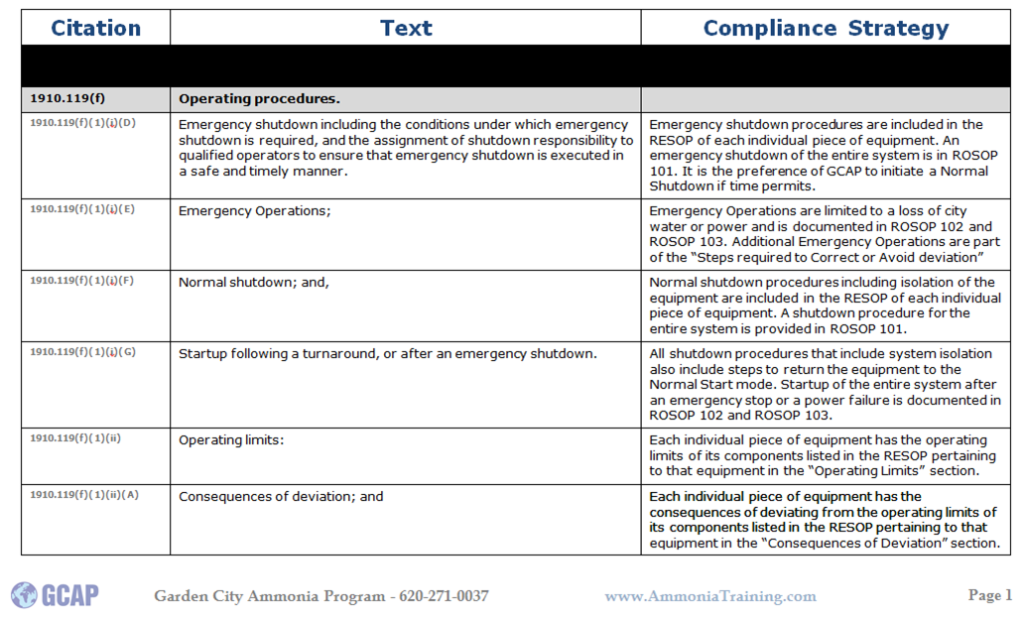
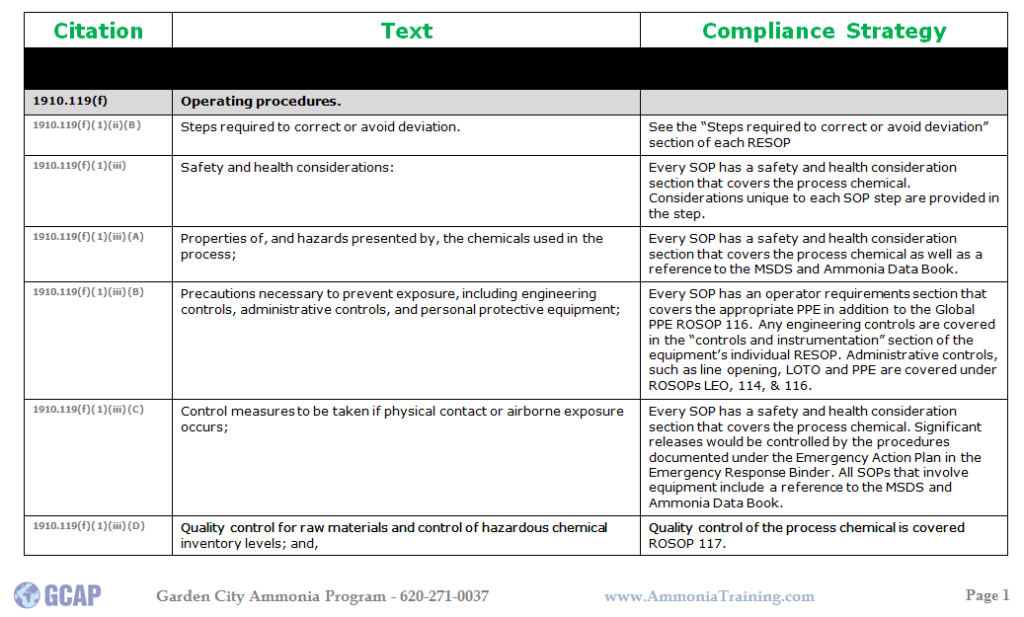
Here is an example for a compliance strategy for SOPs: If you would like some information on GCAP and opportunities for 3rd parties PSM/RMP and PSM/RMP/CalARP Compliance Audits or GAP Analysis please give us a call @ 620.271.0037